Parameters#
Continuing with the example of OPLS from the previous section (Functional Form) let’s recap the equation representing the energy as a function of the atomic coordinates:
The coordinates of the atoms enter this through the distances between atoms, \(r_{ij}\) and the torsional (or dihedral) angles, \(\phi\). There are also a number of parameters in the equation: \(A_{ij}\), \(B_{ij}\), \(V_{t,0}\), \(V_{t,1}\), \(V_{t,2}\), and \(V_{t,3}\). In this section we will continue our journey through the OPLS paper, seeing how OPLS defines these parameters.
We will work through the parameters in the order they appear in the Equation (1) above. This is different than the order that they present them in the paper.
Intermolecular Lennard-Jones Parameters#
The Lennard-Jones parameters for the intermolecular interactions are given in Table III:
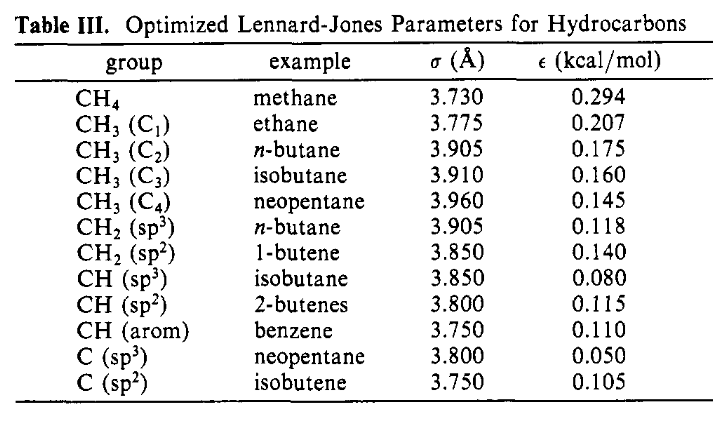
Figure 1: The Intermolecular Lennard-Jones Parameters#
It is a bit confusing that they use the parameters \(A_{ij}\) and \(B_{ij}\) in Equation (1) but then present the values for a different form of the equation for the Lennard-Jones potential:
Just realize that the two forms are equivalent, and there are simple equations to transform between them.
There is one more detail. Note that in the equation the parameters have two subscripts, e.g. \(\epsilon+{ij}\) but those listed in their Table III are for a single (united) atom, i.e. they have a single index \(\epsilon+{i}\). This is common in forcefields, and is where the combining rules come into play, as was mentioned in the Functional Form section. OPLS uses the geometric combining rules:
or equivalently
Intramolecular Lennard-Jones Parameters#
Now that we have understood the intermolecular LJ parameters, let’s move on to the intramolecular ones, which turn out to be much simpler:
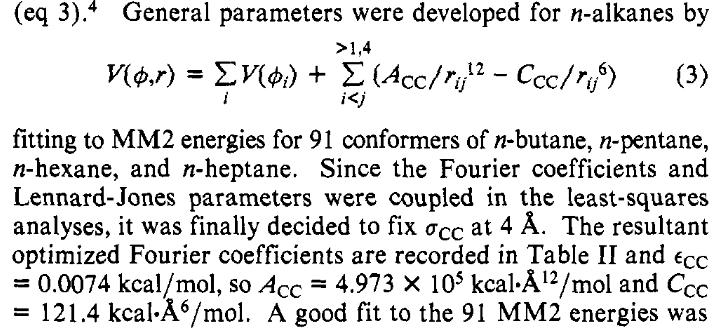
Figure 2: Intermolecular Lennard-Jones parameters.#
OPLS uses a single set of parameters for the intramolecular Lennard-Jones terms:
These can be used as-is in Equation (2).
This coompletes the Lennard-Jones parameters for OPLS.
Torsion Parameters#
There are four parameters for each type of torsion in the truncated Fourier expansion in Equation (1) which are given in Table II:
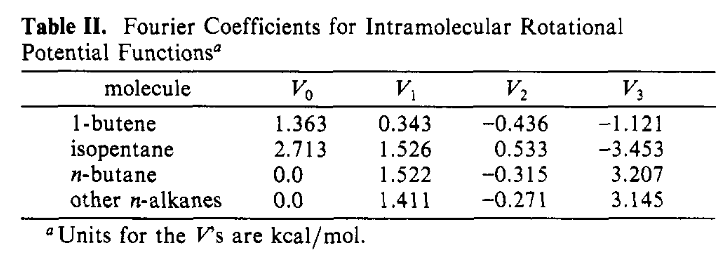
Figure 3: The parameters for the torsions.#
The torsion parameters are straightforward: after determining the type of the torsion – whether it is the single torsion in 1-butene, isopentane, or n-butane, or it is any other n-alkane – the values are entered as-is into the equation.
Standard Bond Lengths and Angles#
Equation (1) does not explicitly mention the bond lengths and angles because they do not affect the energy; however, the bond lengths and angles need to be constrained to the values given in Table I:
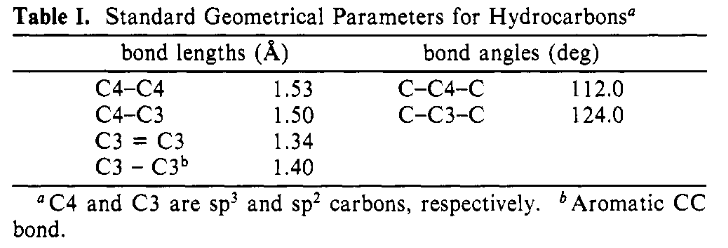
Figure 4: The standard bond lengths and angles.#
Summary#
We have now found and understood all of the parameters included in the original OPLS paper. The paper is a bit confusing in three main parts:
Using one form of the Lennard-Jones equations in the functional form, but providing the parameters for a different form without much comment. This is not an issue if you know that there are two equivalent forms of the equation and that it is easy to convert the parameters from one form to the other.
Only very briefly mentioning the combining rules used. This is increasinlgy common in papers about forcefields, but until you realize that almost all forcefields and programs use combining rules to get the values of \(\epsilon_{ij}\) from \(\epsilon_{i}\) and \(\epsilon_{j}\) it is not obvious where the parameters with two indices come from.
The way that the intramolecular Lennard-Jones terms are presented in combination with the torsion terms, which creates two different equations for torsions, is confusing until you realize that the first, simpler equation is only applicable to molecules with atoms no further appart than 1,4. For these molecules, there are no terms in the sum over atoms for Lennard-Jones terms in the second, more complex equation so the two equations are in practice equivalent. Finally, by removing the Lennard-Jones terms from the torsion energy and combining them with the intermolecular Lennard-Jones terms the overall equiations are simplified and streamlined to a great extent.
Handling these issues made the discussion in this section longer than it needed to be. The final summary is quite simple:
There are two sets of Lennard-Jones parameters, one set for the intermolecular interactions and another simple set for all intramolecular interactions, wich are limited to atoms that are 1,5 to each other or further apart.
There are only four sets of torsion parameters: one for the single torsion in each of 1-butene, isopentane, and n-butane and a final set for all the other torsions in normal alkanes.
The bond lengths and angles are rigidly fixed at the length or angle given in their table 1.
Later in the development of forcefields most evolved to a simpler, less specialized description. For example, there is typically only one set of Lennard-Jones parameters, which are applied to all atoms seprated by either three or four bonds, or more. Most forcefields also moved away from united-atoms representations where the hydrogen atoms were “swallowed” by the carbon atoms they were attached to. In addition, rigid bonds and angle largely gave way to flexible models except for water itself.